Abstract
Chromosomal translocations involving the MLL1 gene often drive infant acute myeloid leukemia (AML). MLL fusion proteins (e.g., MLL-ENL, MLL-AF9, MLL-AF10) activate self-renewal programs in hematopoietic stem and progenitor cells, ultimately leading to transformation. The high frequency of MLL1 rearrangements in infant leukemias suggests that neonatal progenitors are uniquely poised to transform in response to these mutations. Indeed, we have shown that MLL-ENL initiates AML more efficiently in neonatal progenitors than in adult progenitors (Okeyo-Owuor et. al. Blood Advances, 2019). This raises the question of whether MLL-ENL induces key effectors of transformation more efficiently in neonatal progenitors than in adult progenitors. We identified Ski/Dach Domain Containing 1 (Skida1) as a gene that is highly induced by MLL-ENL in neonatal, but not adult hematopoietic progenitors. SKIDA1 is also highly expressed in human pediatric AML, and this expression is largely restricted to leukemias with MLL1 rearrangements. These observations suggest that Skida1 may be a critical, neonate-specific effector of MLL-ENL driven leukemogenesis.
To test whether SKIDA1 is necessary for normal hematopoiesis, we generated mice with Skida1 germline loss-of-function and conditional loss-of-function alleles. We characterized normal hematopoiesis by flow cytometry at embryonic day 16.5 (E16.5), postnatal day 0 (P0), P14, and 8-10 week-old adult mice. Hematopoietic stem cell (HSC), multipotent progenitors (MPP), and more committed progenitors cell numbers were not perturbed. We then assessed HSC function by transplanting HSCs into lethally irradiated mice to assess their multi-lineage repopulating potential. Loss of Skida1 did not perturb HSC function at any stage of development. These data indicate that Skida1 does not play a role in normal hematopoiesis, consistent with low level of expression in non-malignant conditions.
We next assessed whether SKIDA1 overexpression by itself is sufficient to promote colony formation and proliferation in hematopoietic progenitors. We ectopically expressed SKIDA1 in normal hematopoietic cells and found that it suppressed, rather than promoted, colony formation. However, ectopic SKIDA1 expression in immortalized 32D hematopoietic cells enhanced cell growth. Thus, SKIDA1 expression may enhance pre-leukemic cell growth provided other mechanisms exist to mitigate differentiation.
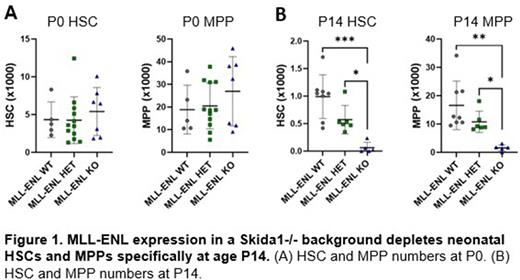
Next, we tested whether Skida1 modulates HSC/MPP numbers in the context of MLL-ENL expression. We crossed Skida1-/- mice to mice with a tetracycline inducible MLL-ENL transgene. The resulting mice expressed MLL-ENL specifically in hematopoietic cells beginning at E10.5 in the absence of doxycycline, concordant with the onset of Vav1-Cre expression. When we induced MLL-ENL expression in Skida1-/- mice, we observed near complete loss of HSCs and a severe reduction of MPPs (Figure 1). This phenotype emerged between P0 and P14, indicating that it requires a neonatal context to manifest. This coincides with the developmental stage at which MLL-ENL most efficiently induces AML (Okeyo-Owuor et. al. Blood Advances, 2019). The data suggests that Skida1 sustains pre-leukemic MLL-ENL-expressing HSCs and MPPs shortly after birth.
To characterize how SKIDA1 sustains MLL-ENL-expressing progenitor cells at the molecular level, we identified SKIDA1 binding partners. We expressed FLAG-tagged SKIDA1 in various AML and hematopoietic cells lines and performed pull-down assays followed by mass spectrometry. Our data show that SKIDA1 binds Histone H2B, as well as several members of the Polycomb Repressive Complex 2 (PRC2), including EZH1 and EZH2. While additional mechanistic studies are required to dissect these interactions further, the data suggest that SKIDA1 promotes MLL-ENL-driven AML by modulating PRC2 activity.
Our current work raises two important points. First, SKIDA1 appears to have a far more important role in sustaining MLL-ENL-expressing progenitors than normal progenitors, raising the prospect for an excellent therapeutic window. Second, our findings show that age-specific MLL-ENL targets can shape pre-leukemic hematopoiesis and potentially AML initiation. This may help explain why MLL1 rearrangements account for a higher percentage of infant and childhood leukemias than adult leukemias.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal